Optimize Haptic Feedback with Specialty Gels & Fluids
As new parenteral drug products are developed, they often require easy-to-use, precise, consistent, portable, safe, and stable devices to deliver the drug into the body. Carefully designed, innocuous, and precisely controlled lubricants and motion control (damping) gels from Nye assist device designers and manufacturers confronted with functionality and usability challenges related to the control of motion and friction. One of those challenges may be to optimize haptic feedback, especially in hand-held devices, to satisfy the goals of device optimization related to Human Factors design requirements.
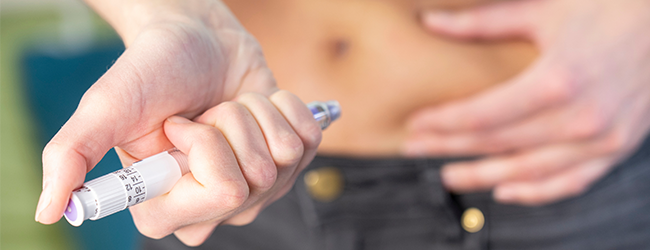
For example, drug delivery devices may have dials that enable the patient to set their dosage levels, and many devices have a mechanical trigger or button to start the drug delivery process. Other events involve sounds, lights or level indicators which alert the user that devices are in delivery mode, or that is has completed the cycle. As mechanical or electromechanical energy is released inside a device during delivery, sound or vibration must be deadened to put the patient at ease. These design factors improve drug product compliance and healthcare outcomes, saving money and time for health service organizations.
How Can Gels & Fluids Optimize Haptic Feedback?
A viscoelastic gel or fluid can be used to ensure the mechanical design imparts desirable haptic feedback while eliminating mechanical shock, noise or vibration that may worry the patient or caregiver. The rheological properties of a motion control gel can be changed to impart the desired “feel” when moving dials, buttons, or other triggers. Gels and fluids can also minimize any vibrations that may be felt by the patient.
Ensure Consistent Performance
The supply chain presents challenges for drug delivery devices such as:
- Long shelf-life
- Shock and vibration
- Temperature variation
Many parenteral drug products must be stored at low temperature which yields an additional risk as patients might use the device before it has warmed to the correct temperature range. In warmer climates, devices may be actuated above normal room temperatures. We’ll select or develop the right gel or fluid that optimizes mechanism functionality while also staying in its intended location, remaining structurally stable over time and temperature, and displaying appropriate materials compatibility.
Partner with the Industry Experts!
With a breadth of experience in this industry, a choice of solutions, and extensive tribological, physical and rheological testing capabilities, Nye’s Research and Development team are ready to partner with you. Our projects are discretely and effectively managed and our ISO 13485:2016 QS certification ensures that partners receive the specification development, documentation and audit support they need, as well as batch-to-batch consistency. We’ll assist from concept to the industrialization phase of your device, and beyond.
Want to learn more? Contact us to let us know how we can help with your application.

