Grease Base Oil Compatibility
Some lubricants can “attack” certain plastics and elastomers. The base oil can infiltrate the solid material or cause the solid’s components to leach into the lubricant.

The material compatibility of specific plastics and elastomers should always be tested by evaluating physical properties of your component such as tensile strength, dimensional stability, and gravimetric stability after immersion in the lubricant. Higher temperatures and lower base oil viscosities usually exacerbate chemical incompatibility.
Below is a chart that highlights the compatibility of synthetic base oils with common design materials. Testing should always be conducted to validate compatibility.
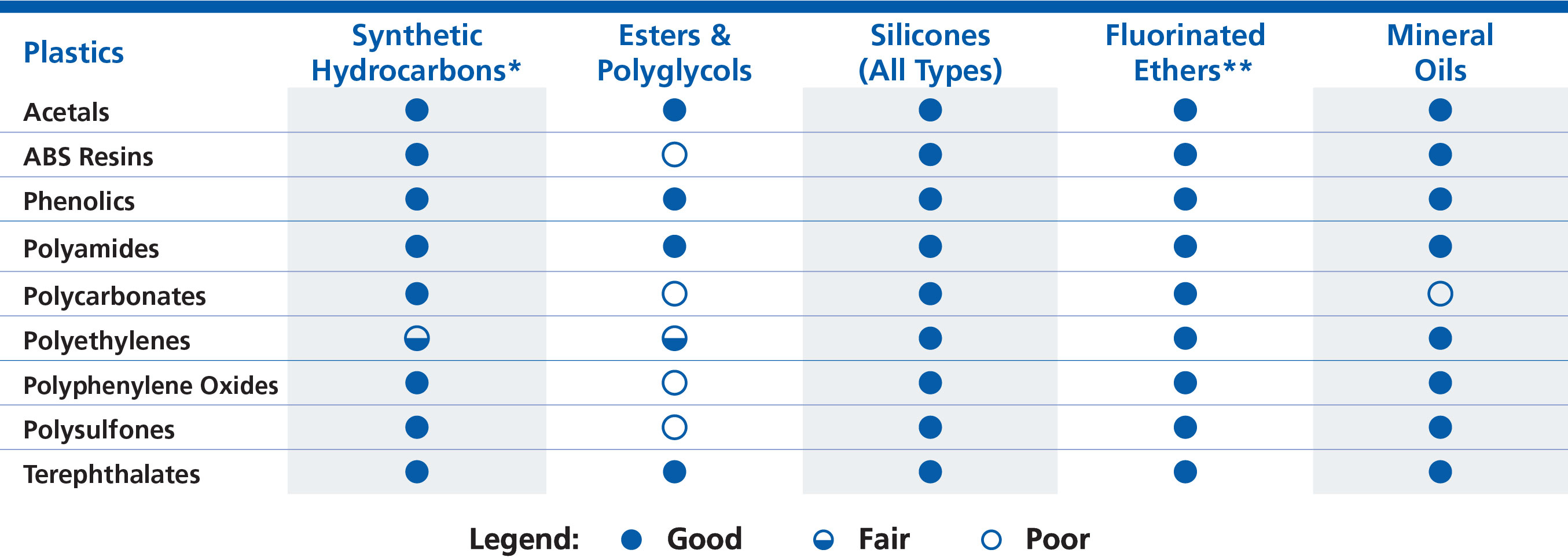
Plastics
Most plastics are compatible with synthetic hydrocarbon base oils, multiplyalkylated cyclopentanes, silicone oils, and PFPE oils. ABS, polycarbonate, polyester, PPO, polysulfone, and PVC are usually only impacted by polar base oils, such as esters. Acetal, phenolic, polyamide-imide, polyamide, polyetherimide, polyimide, polytetrafluoroethylene, and polybutylene terephthalate tend to be robust plastics in many aspects including mechanical strength and thermal stability, as well as being compatible with all base oils
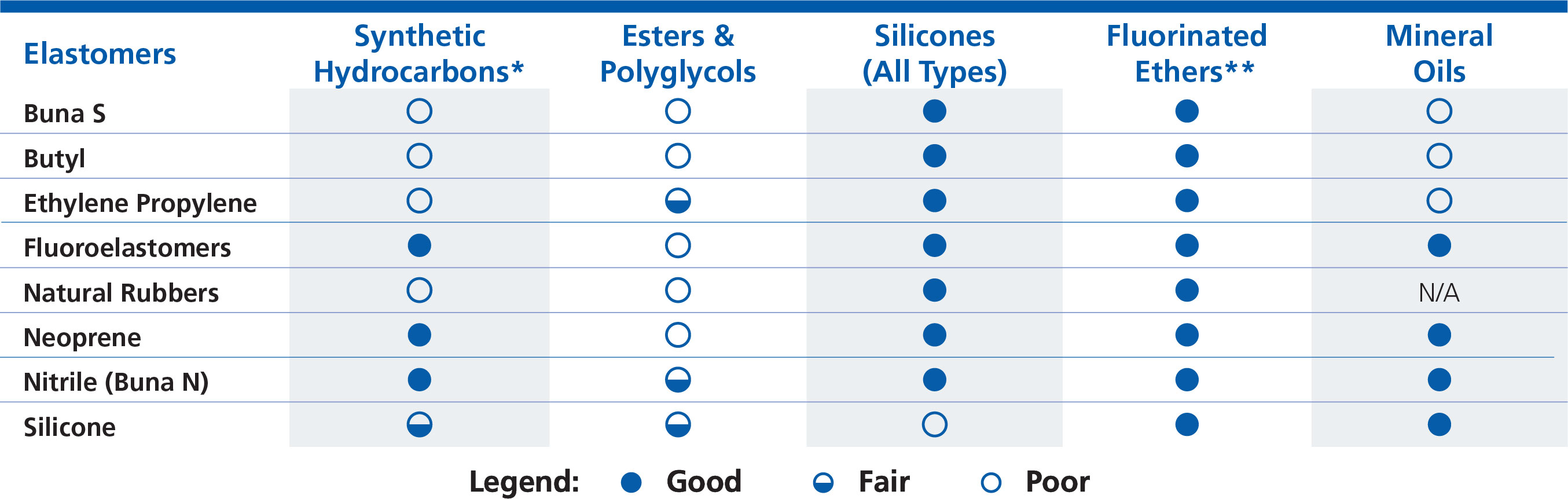
Elastomers
In general, SBR, Butyl, and natural rubber are more susceptible to base oil incompatibilities where as silicones are unaffected by anything other than silicone oils. Fluoroelastomers are highly chemically resistant. Some materials, like neoprene and nitrile, have chemical structures similar to those found in SBR, Butyl, and natural rubber etc. but with functional groups that offer more protection against synthetic hydrocarbon based oils, but are still susceptible to more aggressive, polar base oils such as esters.
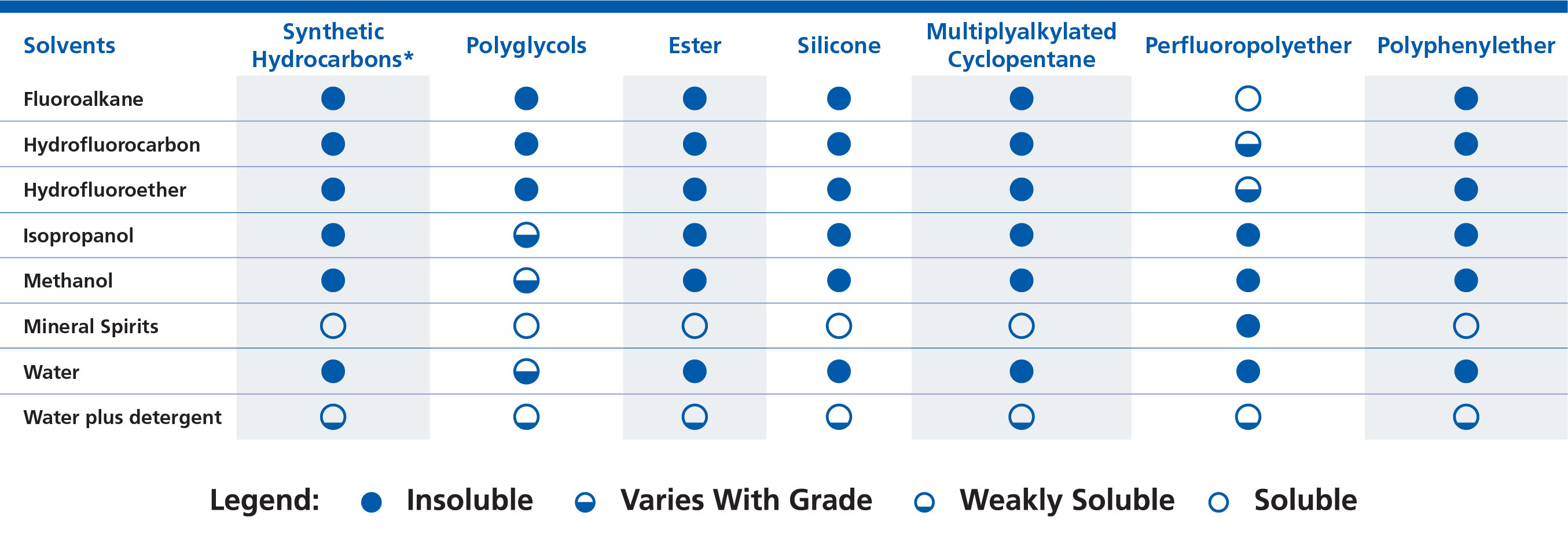
Solvents
In the case of solvents, base oil compatibility depends largely on solubility. If a base oil is soluble in a solvent, then that means it can be diluted and/or washed away, essentially removing the lubricant from the desired area. When considering solubility, the general principle is “like dissolves like” so solvents and base oils that have similar properties to each other (usually polarity) are more likely to be soluble than those that have different properties from each other. For example, the fluorine containing perfluoropolyether base oil is most easily dissolved by fluorine containing solvents, such as fluoroalkanes, hydrofluorocarbons, and hydrofluoroethers.
*Synthetic Hydrocarbons include PAO. **Fluorinated Ethers include PFPE and PPE.


